Efficient Process Control for Biopharmaceutical Manufacturing
What happens if a batch in biopharmaceutical manufacturing fails? Quite simply a lot of resources – human, material, and monetary – are lost instantly. Furthermore, failing a batch leads to a loss of millions of dollars in revenue for a biopharma company, in terms of lost sales. Especially after the COVID-19 pandemic, there is constant pressure on the biopharmaceutical industry to increase productivity cost-effectively while maintaining the highest standards of process and product quality. Overcoming this challenge in such a complex environment as biopharmaceutical manufacturing is a demanding task.
Facing the need for better productivity in bioprocesses, biopharma companies are searching for powerful technologies to gain profound process understanding. Process efficacy is also a significant motivation for many biopharma companies to design continuous process control and predictive models for feed control.
Data-driven Process Controlling
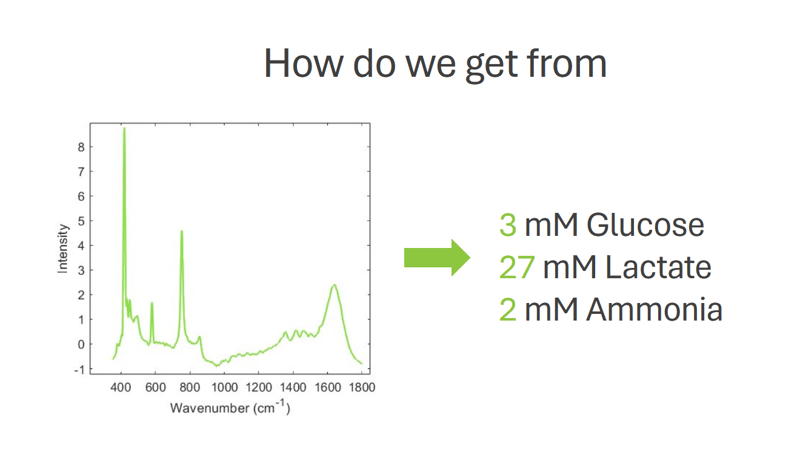
There is a severe need for efficient analytical technologies in biopharmaceutical manufacturing. The process is extremely intricate, and process failures happen more frequently than desired. One of the challenges has been a lack of relevant data. Until now, the process attributes for monitoring have been mostly simple variables such as pH, temperature, and dissolved oxygen. However, efficient and successful process control in an environment of living organisms requires extensive real-time process data, including a wide variety of chemical and biological variables like:
• Concentration of nutrients like glucose, glutamine, and glutamate
• Concentration of metabolites like lactate
• Amino acids
• Proteins
• Cell viability
• Biomass
All this data is now available and can be harnessed to enhance the process and product quality by using modern Process Analytical Technology (PAT) tools. Adopting PAT tools for real-time process monitoring provides a thorough understanding of what is happening in the process, ensuring that no critical events are missed. Raman spectroscopy, particularly time-gated Raman spectroscopy, has the leading role among the various PAT tools available.
Tools for Controlling the Entire Process from Development to Production
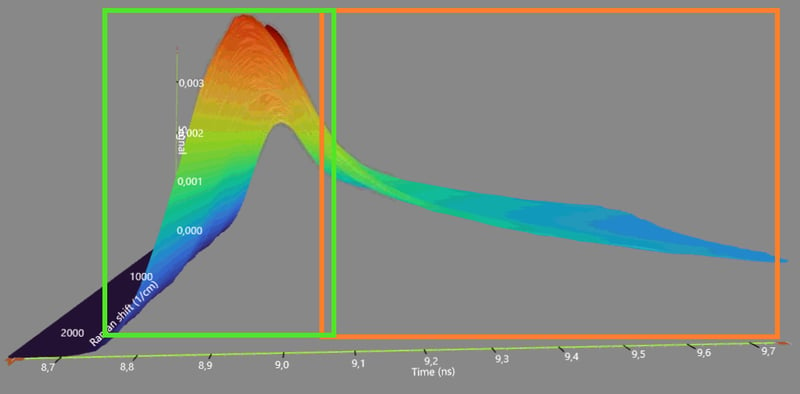
The reputation of Raman spectroscopy has not been appreciated in history. This is mainly because of a physical phenomenon called fluorescence that tends to obscure the Raman spectrum. Despite the fluorescence interference conventional Raman has been widely used in the biopharma industry. Gradually, a transition to time-gated Raman tools is occurring. Time-gated Raman spectroscopy has mostly solved the challenge by suppressing the fluorescence, which significantly improves the quality of the Raman spectrum.
Raman tools have been used only in the development phase of biopharmaceuticals. But currently, the first biopharma companies are adopting Raman-based tools for production as well.
There were several interesting cases of transferring Raman tools to production that were described in the Bioprocess International Conference (Boston, September 2023). For example, Merck stated that they have adopted a wide variety of PAT tools to design continuous bioprocess control for process efficacy. Particularly for bioreactor measurements and monitoring in production, they have chosen Raman because it’s beneficial not only in improved process productivity but also in consistent product quality and flexibility.
In their presentation, Merck mentioned that they even developed Raman-based models for glucose and lactate profiles in the process to predict feed control. With Raman-based modeling, they were able to increase the productivity of biologics from 1,7 g/l to 5 g/l. Thus, in this case, the yield was tripled. This is a strong demonstration of how powerful analytical tool Raman spectroscopy is for increased productivity and flexibility.
The story of Merck illustrates the power of Raman spectroscopy as an analytical tool for continuous process monitoring and predictive feed controlling. Integration of advanced technologies such as time-gated Raman spectroscopy into biopharmaceutical manufacturing processes provides even more influential opportunities to upgrade the processes towards enhanced productivity, as well as consistent product and process quality in the industry.
Read more about Timegated® Technology for the biopharmaceutical industry, including benefits, pilots, and a reference case.
Author
 This blog was written by Timegate Instruments’ Head of Marketing Minna Lappi. Minna has an extensive background in the healthcare industry, including leading a service department in an international software corporation. She holds an M.Sc. in Economics, Marketing.
This blog was written by Timegate Instruments’ Head of Marketing Minna Lappi. Minna has an extensive background in the healthcare industry, including leading a service department in an international software corporation. She holds an M.Sc. in Economics, Marketing.